
What disease has infected millions of people, killing an estimated 1.5 million people a year, without drawing a fraction of the attention of the COVID-19 pandemic? The answer is tuberculosis (TB). New, more effective vaccines are needed to reduce the morbidity and mortality of TB, fight the rising threat of AMR, and address inequities in disease burden and economic impact.
What disease has infected millions of people, killing an estimated 1.5 million people a year, without drawing a fraction of the attention of the COVID-19 pandemic? The answer is tuberculosis.
Tuberculosis (TB) is a leading cause of infectious disease deaths worldwide—a person dies from TB every 20 seconds 1. According to the World Health Organization (WHO), TB is currently “the second leading infectious killer after COVID-19.”2. TB has the heaviest impact on the world’s most poor and vulnerable populations, worsening existing inequalities: More than 95% of tuberculosis cases occur in low- and middle-income countries (LMICs), with an estimated two-thirds of total cases occurring in just eight high-burden countries3. In 2021, there were an estimated 10.6 million active TB cases, including 1.2 million cases of TB among children3. Those who survive the disease often experience economic hardship and long-term health impacts4,5.

The Bacille Calmette-Guérin (BCG) vaccine is the only vaccine currently available to protect against TB. BCG has been in use for a century and provides critical protection to 100 million newborns globally each year6. While the BCG vaccine provides good protection for young children, the vaccine’s efficacy wanes throughout the lifespan, providing negligible protection to those over 5 years old6. TB mainly affects adults, leaving millions vulnerable to the devastating effects of this vaccine-preventable disease7. To end the TB epidemic, it is critical to develop vaccines that are effective against TB in all age groups.
The Non-Specific Benefits of the BCG Vaccine: Protection Beyond TB
Despite its inability to protect adults from TB, BCG is a life-saving vaccine for infants and children under five. Beyond protecting against TB in early childhood, evidence suggests the BCG vaccine may also protect infants against other infections, ultimately reducing all-cause mortality8–10 in some contexts. Studies have found that BCG is one of the few vaccines providing additional immunity beyond the target pathogen, called non-specific specific effects or heterologous effects.
- In a series of studies of low birthweight newborns in Guinea-Bissau, administering the BCG vaccine at birth was associated with a 38% reduction in all-cause mortality within the four weeks after birth11.
- A 2023 meta-analysis examined 16 studies conducted among children and adults in both low- and high-income settings12. BCG vaccination was associated with a 44% lower risk of non-TB respiratory infections, a 33% reduction in infection-related mortality, and a 38% reduction in sepsis-related mortality.

Researchers don’t fully understand how BCG offers this broad protection, but one possible explanation is trained immunity — when immunization against one infectious agent can influence a person’s immune response to subsequent infection(s) by an unrelated infectious agent13. Another theory is that BCG immunization can influence an infant’s body response to subsequent routine immunizations8. It’s also possible that these benefits are affected by the timing of vaccination, as research suggests that the BCG vaccine should be given within the first month of life14. More research is needed to fully understand the mechanisms behind these non-specific effects and to determine the optimal timing and dosing for maximum health benefits.
TB and Antimicrobial Resistance: A Growing Health Security Crisis
TB is a major contributor to the global burden of antimicrobial resistance (AMR), or the ability of disease-causing microorganisms (such as bacteria and viruses) to become resistant to drugs and treatment15. Killing 700,000 people each year, AMR is considered a major global public health threat and by 2050 is projected to cause more deaths than cancer16. The rise of multidrug-resistant TB (MDR-TB) is an emerging threat to global health security, with the majority of cases going undetected17.
- Drug-resistant TB accounts for approximately 1 in 3 deaths attributable to AMR18.
- In 2021, there were an estimated 450,000 cases of MDR-TB globally, an increase of approximately 3% since 20203. These accounted for 3.6% of new cases of TB and nearly 1 in 5 of those previously treated.
- Drug-resistant TB accounts for approximately 1 in 3 deaths attributable to AMR18.
- MDR-TB can require up to two years of treatment, including eight months of daily injections, and an estimated 14,000 pills over the course of the treatment18.
- The length and complexity of treating MDR-TB substantially increases health care costs. For example, it costs nearly 25 times more to treat MDR-TB than drug-susceptible TB in South Africa, contributing to a disproportionate portion of the country’s TB budget19.

The fight against AMR and MDR-TB will require a multi-pronged approach, and it will not be easy. TB vaccines can help by reducing the incidence and transmission of TB, which would in turn reduce the need for antimicrobial treatment and help to slow the emergence of AMR. Because vaccines prevent infections in the first place, they play an indispensable role in combatting the global crisis of drug resistance20.
An Economic Case for TB Vaccines
Research indicates that like other immunizations, BCG vaccination is generally cost-effective, particularly in high-incidence settings21. However, these cost savings are not passed downstream to the families affected by TB; treatment for the disease can take months and can lead to catastrophic health costs for families. This is one reason why preventing TB is an important consideration for equity: TB is most likely to impact those who will have the greatest challenges covering the costs of treatment, transportation to a health center, and lost wages. Low-income populations are generally at a higher risk of developing TB, possibly because they have higher exposures to risk factors such as living and working in crowded and poorly ventilated spaces and less access to health care 22. As a result, they are more likely to be saddled with the catastrophic treatment costs of TB.
- According to a review of national patient cost surveys from 23 countries, the percentage of households affected by TB experiencing catastrophic costs ranged from 13–92%, with a pooled average of 47%23. This means that globally, nearly one in two families affected by TB will spend more than 20% of their household income on treatment, a catastrophic expense for many families.
- Research in Ghana demonstrates that TB costs can push households below the poverty line, particularly for those already living in the middle or lower half of the income distribution24.
- The financial impact of TB continues after treatment. Among families participating in a study in South Africa, 35% of previously employed mothers stopped working to care for children who had permanent disabilities from surviving tuberculosis meningitis 25. Nineteen percent of families reported financial loss as a result of caring for children who were disabled by the disease.
The WHO End TB Strategy outlines eliminating the number of TB-affected families facing catastrophic costs as one of its goals, and there is clearly work to be done to meet this ambitious goal26.
“The development and roll-out of new TB vaccines could yield health and economic benefits on a similar scale to some of the most influential health interventions in poorer countries in recent years.”27
Developing new TB vaccines that protect adolescents and adults will require a significant investment, but health economists project that these vaccines will save money in the long run, thanks to averted treatment costs and the boost to the economy associated with a healthy workforce. These vaccines are also expected to advance health equity, as the benefits of new TB vaccines are expected to provide the greatest benefits for those who currently bear the highest disease burden.
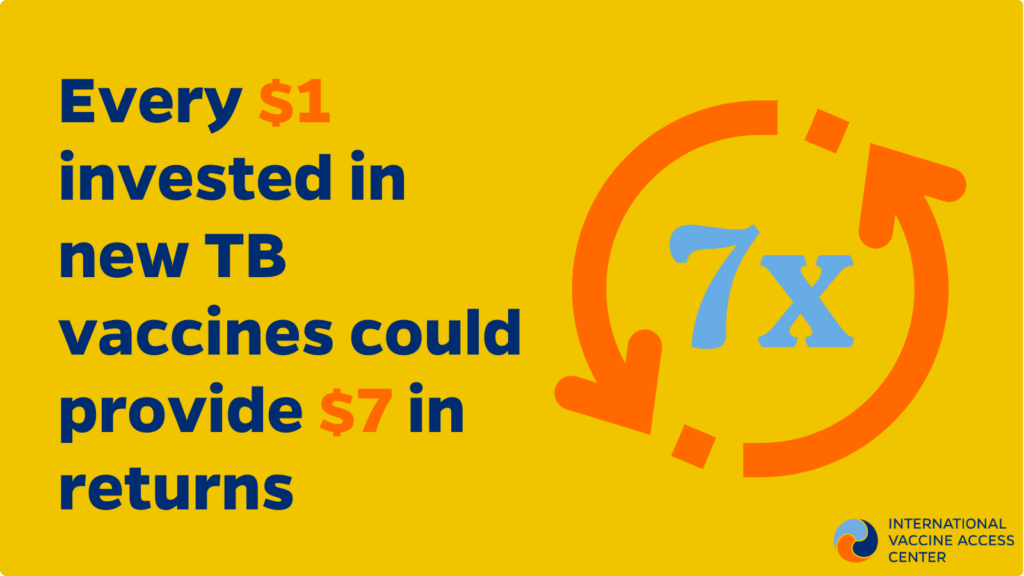
- A 2023 modelling study estimates that a TB vaccine for adolescents and adults would be cost-effective in 70% of settings and could save up to US$474 billion by 205028. In this scenario, every US$1 invested in TB vaccines would provide US$7 in returns.
- A second modelling study29 shows that more than half of TB cases averted by a new vaccine would be among the two poorest income quartiles. These two income quartiles would also account for 46% of averted treatment costs, as well as 66% of cases of catastrophic costs.

Looking Ahead: The Future of TB Vaccines
New, more effective vaccines are needed to reduce the morbidity and mortality of TB, fight the rising threat of AMR, and address inequities in disease burden and economic impact. The BCG vaccine does not adequately protect older children, adolescents, and adults against TB, and continuing to neglect these populations will only exacerbate this growing crisis.
There are at least 16 new TB vaccines currently in development, but significant funding is needed to push these new vaccines through the research pipeline. It is estimated that it will take US$790 million per year to advance TB vaccines, though the average annual investment for the past several years has been just US$115 million30. These vaccines must be prioritized to reduce the burden of this devastating disease and end the TB pandemic.

References
1. Global Pandemic. TB Alliance. Accessed March 16, 2023. https://www.tballiance.org/why-new-tb-drugs/global-pandemic
2. Tuberculosis (TB). Accessed March 16, 2023. https://www.who.int/news-room/fact-sheets/detail/tuberculosis
3. Tuberculosis. Accessed March 16, 2023. https://www.who.int/health-topics/tuberculosis
4. Meghji J, Gregorius S, Madan J, et al. The long term effect of pulmonary tuberculosis on income and employment in a low income, urban setting. Thorax. 2021;76(4):387-395. doi:10.1136/thoraxjnl-2020-215338
5. Shruthi Ravimohan, Hardy Kornfeld, Drew Weissman, Gregory P. Bisson. Tuberculosis and lung damage: from epidemiology to pathophysiology | European Respiratory Society. Accessed March 16, 2023. https://err.ersjournals.com/content/27/147/170077
6. Martinez L, Cords O, Liu Q, et al. Infant BCG vaccination and risk of pulmonary and extrapulmonary tuberculosis throughout the life course: a systematic review and individual participant data meta-analysis. Lancet Glob Health. 2022;10(9):e1307-e1316. doi:10.1016/S2214-109X(22)00283-2
7. 2.1 TB incidence. Accessed March 16, 2023. https://www.who.int/teams/global-tuberculosis-programme/tb-reports/global-tuberculosis-report-2022/tb-disease-burden/2-1-tb-incidence
8. Ritz N, Mui M, Balloch A, Curtis N. Non-specific effect of Bacille Calmette-Guérin vaccine on the immune response to routine immunisations. Vaccine. 2013;31(30):3098-3103. doi:10.1016/j.vaccine.2013.03.059
9. Moorlag SJCFM, Arts RJW, van Crevel R, Netea MG. Non-specific effects of BCG vaccine on viral infections. Clin Microbiol Infect. 2019;25(12):1473-1478. doi:10.1016/j.cmi.2019.04.020
10. Biering-Sørensen S, Jensen KJ, Monterio I, Ravn H, Aaby P, Benn CS. Rapid Protective Effects of Early BCG on Neonatal Mortality Among Low Birth Weight Boys: Observations From Randomized Trials. J Infect Dis. 2018;217(5):759-766. doi:10.1093/infdis/jix612
11. Biering-Sørensen S, Aaby P, Lund N, et al. Early BCG-Denmark and Neonatal Mortality Among Infants Weighing <2500 g: A Randomized Controlled Trial. Clin Infect Dis. 2017;65(7):1183-1190. doi:10.1093/cid/cix525
12. Trunk G, Davidović M, Bohlius J. Non-Specific Effects of Bacillus Calmette-Guérin: A Systematic Review and Meta-Analysis of Randomized Controlled Trials. Vaccines. 2023;11(1):121. doi:10.3390/vaccines11010121
13. Covián C, Fernández-Fierro A, Retamal-Díaz A, et al. BCG-Induced Cross-Protection and Development of Trained Immunity: Implication for Vaccine Design. Front Immunol. 2019;10. Accessed March 16, 2023. https://www.frontiersin.org/articles/10.3389/fimmu.2019.02806
14. Berendsen MLT, Smits J, Netea MG, Ven A van der. Non-specific Effects of Vaccines and Stunting: Timing May Be Essential. eBioMedicine. 2016;8:341-348. doi:10.1016/j.ebiom.2016.05.010
15. Antimicrobial resistance. Accessed March 16, 2023. https://www.who.int/news-room/fact-sheets/detail/antimicrobial-resistance
16. Tagliabue A, Rappuoli R. Changing Priorities in Vaccinology: Antibiotic Resistance Moving to the Top. Front Immunol. 2018;9. Accessed March 16, 2023. https://www.frontiersin.org/articles/10.3389/fimmu.2018.01068
17. Kenyon T. Tuberculosis Is A Threat To Global Health Security. Health Aff (Millwood). 2018;37(9):1536-1536. doi:10.1377/hlthaff.2018.0894
18. Antimicrobial Resistance. TB Alliance. Published November 17, 2016. Accessed March 16, 2023. https://www.tballiance.org/why-new-tb-drugs/antimicrobial-resistance
19. Pooran A, Pieterson E, Davids M, Theron G, Dheda K. What is the Cost of Diagnosis and Management of Drug Resistant Tuberculosis in South Africa? PLoS ONE. 2013;8(1):e54587. doi:10.1371/journal.pone.0054587
20. 160525_Final paper_with cover.pdf. Accessed March 16, 2023. https://amr-review.org/sites/default/files/160525_Final%20paper_with%20cover.pdf
21. Machlaurin A, Pol S van der, Setiawan D, van der Werf TS, Postma MJ. Health economic evaluation of current vaccination strategies and new vaccines against tuberculosis: a systematic review. Expert Rev Vaccines. 2019;18(9):897-911. doi:10.1080/14760584.2019.1651650
22. Lönnroth K, Jaramillo E, Williams BG, Dye C, Raviglione M. Drivers of tuberculosis epidemics: the role of risk factors and social determinants. Soc Sci Med 1982. 2009;68(12):2240-2246. doi:10.1016/j.socscimed.2009.03.041
23. 6.2 National cost surveys. Accessed March 16, 2023. https://www.who.int/publications/digital/global-tuberculosis-report-2021/uhc-tb-determinants/cost-surveys
24. Pedrazzoli D, Siroka A, Boccia D, et al. How affordable is TB care? Findings from a nationwide TB patient cost survey in Ghana. Trop Med Int Health TM IH. 2018;23(8):870-878. doi:10.1111/tmi.13085
25. Krauss-Mars AH, Lachman PI. Social factors associated with tuberculous meningitis. A study of children and their families in the western Cape. South Afr Med J Suid-Afr Tydskr Vir Geneeskd. 1992;81(1):16-19.
26. The End TB Strategy. Accessed March 16, 2023. https://www.who.int/teams/global-tuberculosis-programme/the-end-tb-strategy
27. New TB vaccines could produce substantial health and economic benefits in coming decades | Gavi, the Vaccine Alliance. Accessed March 16, 2023. https://www.gavi.org/vaccineswork/new-tb-vaccines-could-produce-substantial-health-and-economic-benefits-coming
28. The cost and cost-effectiveness of novel tuberculosis vaccines in low- and middle-income countries: A modeling study | PLOS Medicine. Accessed March 16, 2023. https://journals.plos.org/plosmedicine/article?id=10.1371/journal.pmed.1004155
29. Portnoy A, Clark RA, Weerasuriya CK, et al. The potential impact of novel tuberculosis vaccines on health equity and financial protection in low- and middle-income countries. Published online October 29, 2022:2022.10.29.22281678. doi:10.1101/2022.10.29.22281678
30. An investment case for new tuberculosis vaccines. Accessed March 16, 2023. https://www.who.int/publications-detail-redirect/9789240064690
