HPV Vaccination: A Critical Cancer Prevention Tool
Since 2006, vaccines have been available to protect against human papillomavirus (HPV), an extremely common infection that causes virtually all cases of cervical cancer – the fourth most common cancer in women globally – and is also a known cause of several other types of cancer that impact both men and women [1]. In most instances, the immune system can clear HPV without any need for treatment, but persistent infection with high-risk strains of HPV can lead to several types of cancer [2]. This leads to stark disparities in cancer burden for women living with HIV, whose compromised immune systems may not clear HPV infections as readily; these women are six times more likely to develop cervical cancer [3]. Geographical disparities in cervical cancer burden also persist. In 2020, more than 600,000 women around the world were diagnosed with cervical cancer, with up to 90% of new global cases occurring in low- and middle-income countries (LMICs) and the majority of cancer cases and deaths concentrated in sub-Saharan Africa, Central America, and Southeast Asia [2]. To address these inequities and reduce global disease burden, the World Health Organization (WHO) has committed to a global strategy to accelerate the elimination of cervical cancer as a public health problem. Along with screening and treatment, HPV vaccination is a key cornerstone of this strategy–by 2030, WHO aims to vaccinate 90% of girls against HPV by the age of 15 [4].

Although this is an ambitious goal, new evidence-based recommendations from WHO may contribute to increased HPV vaccination coverage, especially where it is needed most. WHO had previously recommended a two-dose schedule for HPV vaccines, but an off-label recommendation for a one-dose schedule was added in December 2022 based on recent efficacy data from single-dose trials [5]. The latest guidance recommends a one or two-dose schedule for girls aged 9–14 as well as those between 15–20-years old, with girls older than 21 and those who are immunocompromised or living with HIV recommended to receive a third dose. This change is expected to have important programmatic and financial implications for HPV vaccination programs, particularly in LMIC settings. Further, a single-dose schedule could be an important tool to improve health equity and reduce disparities in HPV-related cancers, protecting all girls everywhere against this preventable disease.
Evidence for a Single-Dose HPV Schedule
A number of rigorous studies have been conducted to determine the efficacy and immunogenicity of a single-dose regimen of HPV vaccines. Researchers have concluded that a single dose of HPV vaccine provides high levels of protection against high-risk strains of HPV, even several years after vaccination, and induces a robust immune response.

- In a randomized trial in Kenya (KEN SHE), a single-dose of HPV vaccine was found to be 97.5% effective in preventing cancer-causing strains of HPV among 15–20-year-old girls [6]. Researchers examined the efficacy of single-dose bivalent (a single shot that can protect against two strains of a virus) and nonavalent HPV vaccines (a single shot that can protect against nine strains of a virus) among 15–20-year-old girls. After 18 months of follow-up, both the bivalent and nonavalent vaccines demonstrated 97.5% vaccine efficacy against high-risk strains of HPV. Researchers subsequently published results demonstrating similar vaccine efficacy three years following vaccination: bivalent vaccine efficacy remained at 97.5% (95% CI 90.0–99.4%), while nonavalent vaccine efficacy was 98.8% (CI 91.3–99.8%) [7].
- In a Costa Rican study (CVT), a single dose of HPV vaccine was found to provide a similar level of protection (82.1%) against high-risk strains of HPV as two or three doses (83.8% and 80.2%, respectively), even 11 years following vaccination [8]. Researchers examined the dose-specific vaccine efficacy of the bivalent HPV vaccine among 18–25-year-old women and determined that vaccine efficacy against high-risk strains of HPV was high, regardless of the number of doses received. Protection persisted for approximately 11 years following initial vaccination. Vaccine efficacy was 80.2% (95% CI = 70.7% – 87.0%) for three doses, 83.8% (95% CI = 19.5% – 99.2%) for two doses, and 82.1% (95% CI = 40.2% to 97.0%) for a single dose, with no statistically significant differences in either vaccine efficacy or infection rates across the three groups.
- A cohort study in India (IARC India) found that the protection provided by a single dose of HPV vaccine was comparable to that provided by two or three doses, even 10 years after vaccination [9]. Researchers compared the vaccine efficacy of a single dose of quadrivalent HPV vaccine to two and three doses in protecting against high-risk HPV strains. After 10 years of following the cohort of women, there were no significant differences in the frequency of incident HPV infection among adolescent women who received one, two, or three doses of HPV vaccine. The vaccine efficacy of a single dose was found to be 95.4% (95% CI 85.0 – 99.0), which did not differ significantly from the efficacy of two or three doses.
- In Tanzania, a randomized trial among 9–14-year-old girls (DoRIS) found that two years after vaccination, a single dose of HPV vaccine produced a non-inferior immune response for a high-risk strain of HPV compared to two or three doses [10]. Researchers examined the immune response two years after vaccination with a single dose of HPV vaccine. Compared to two or three doses, a single dose of either bivalent or nonavalent HPV vaccine produced non-inferior levels of antibodies against HPV 16. Although non-inferiority was not met for HPV 18 antibodies, at least 98% of girls who received a single dose of HPV vaccine were seropositive for these antibodies two years following vaccination.
Moving to a Single Dose Schedule – Why it Matters
Compared to many of the routine immunizations given to infants and adolescents, HPV vaccination presents unique programmatic, financial, and logistical challenges, many of which could potentially be addressed by the use of a single-dose vaccination schedule.
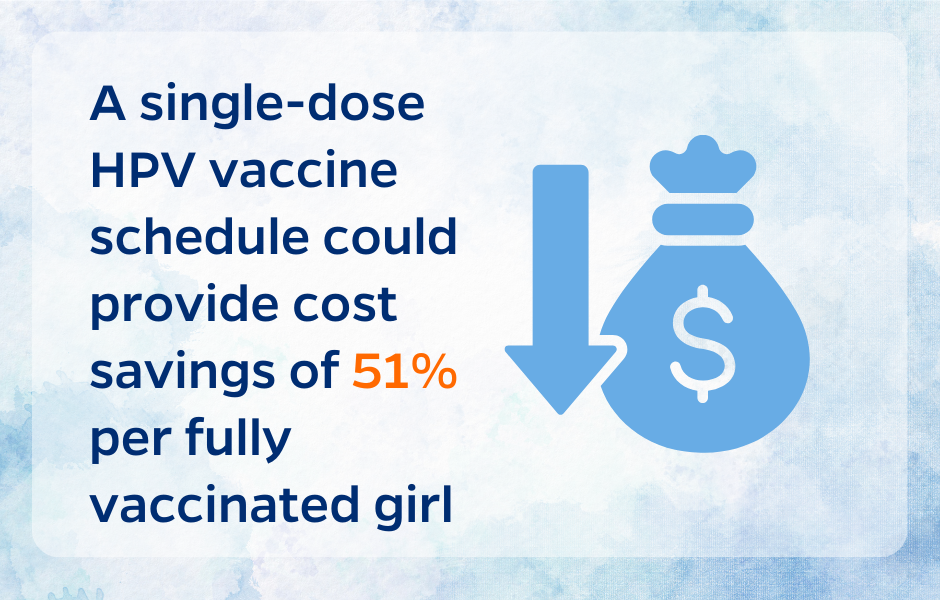
Countries’ limited immunization budgets have put HPV vaccine introduction in direct competition with the introduction of several other life-saving vaccines, including pneumococcal conjugate vaccines and rotavirus vaccines [11]. Compared to a two-dose schedule, a single-dose schedule for HPV vaccines would significantly reduce procurement and delivery costs [11]. For example, an economic study in Tanzania estimated that compared to a two-dose HPV vaccination schedule, a single-dose schedule would reduce the cost per fully vaccinated girl by 51%, accounting for all financial costs including injection supplies, training, and outreach activities [12]. These cost savings could potentially stretch immunization budgets further, allowing countries to protect more girls against HPV and cervical cancer. Additional cost savings may be seen in reduced cold chain requirements, as fewer doses would require countries to purchase fewer refrigerators, for example [13].
Although countries are well-versed in delivering vaccines to children under 5, vaccines targeting older children are more difficult to integrate into existing immunization programs. A single dose of HPV vaccine could be delivered once per year during child health weeks or annual vaccination events, eliminating the need for follow-up and simplifying delivery to this hard-to-reach population [11].
Additional Resources
The resources below provide additional information about HPV vaccination and the evidence for a single-dose schedule.
- VIEW-hub HPV Module: View the latest data on HPV introduction and use for 194 countries through VIEW-hub, IVAC’s open access data visualization tool.
- PATH Single-Dose HPV Vaccine Evaluation Consortium: PATH has gathered data on the value of a single-dose HPV vaccination schedule from clinical trials, observational studies, and modeling analyses.
- CHIC Brief: HPV Dosing Recommendations and Implications: The Coalition to Strengthen the HPV Immunization Community (CHIC) summarizes the latest WHO HPV vaccine dosing recommendations in a two-page brief.
References
1. Sung H, Ferlay J, Siegel RL, et al. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J Clin. 2021;71(3):209-249. doi:10.3322/caac.21660
2. Cervical Cancer. World Health Organization. Updated 17 November 2023. Accessed 25 January 2024. https://www.who.int/news-room/fact-sheets/detail/cervical-cancer
3. Stelzle D, Tanaka LF, Lee KK, et al. Estimates of the global burden of cervical cancer associated with HIV [published correction appears in Lancet Glob Health. 2021 Feb;9(2):e119]. Lancet Glob Health. 2021;9(2):e161-e169. doi:10.1016/S2214-109X(20)30459-9
4. Global strategy to accelerate the elimination of cervical cancer as a public health problem. Geneva: World Health Organization; 2020
5. World Health Organization. Human papillomavirus vaccines: WHO position paper (2022 update) Weekly Epidemiological Record. 16 Dec 2022, No 50, 97, 645-672. https://www.who.int/publications/i/item/who-wer9750-645-672
6. Barnabas RV, Brown ER, Onono M, et al. Single-dose HPV vaccination efficacy among adolescent girls and young women in Kenya (the KEN SHE Study): study protocol for a randomized controlled trial. Trials. 2021;22(1):661. Published 2021 Sep 27. doi:10.1186/s13063-021-05608-8
7. Barnabas RV, Brown ER, Onono MA, et al. Durability of single-dose HPV vaccination in young Kenyan women: randomized controlled trial 3-year results. Nat Med. 2023;29(12):3224-3232. doi:10.1038/s41591-023-02658-0
8. Kreimer AR, Sampson JN, Porras C, et al. Evaluation of Durability of a Single Dose of the Bivalent HPV Vaccine: The CVT Trial. J Natl Cancer Inst. 2020;112(10):1038-1046. doi:10.1093/jnci/djaa011
9. Basu P, Malvi SG, Joshi S, et al. Vaccine efficacy against persistent human papillomavirus (HPV) 16/18 infection at 10 years after one, two, and three doses of quadrivalent HPV vaccine in girls in India: a multicentre, prospective, cohort study [published correction appears in Lancet Oncol. 2022 Jan;23(1):e16]. Lancet Oncol. 2021;22(11):1518-1529. doi:10.1016/S1470-2045(21)00453-8
10. Watson-Jones D, Changalucha J, Whitworth H, et al. Immunogenicity and safety of one-dose human papillomavirus vaccine compared with two or three doses in Tanzanian girls (DoRIS): an open-label, randomised, non-inferiority trial. Lancet Glob Health. 2022;10(10):e1473-e1484. doi:10.1016/S2214-109X(22)00309-6
11. Gallagher KE, LaMontagne DS, Watson-Jones D. Status of HPV vaccine introduction and barriers to country uptake. Vaccine. 2018;36(32 Pt A):4761-4767. doi:10.1016/j.vaccine.2018.02.003
12. Hsiao A, Struckmann V, Stephani V, et al. Costs of delivering human papillomavirus vaccination using a one- or two-dose strategy in Tanzania. Vaccine. 2023;41(2):372-379. doi:10.1016/j.vaccine.2022.11.032
13. Gallagher KE, Kelly H, Cocks N, et al. Vaccine programme stakeholder perspectives on a hypothetical single-dose human papillomavirus (HPV) vaccine schedule in low and middle-income countries. Papillomavirus Res. 2018;6:33-40. doi:10.1016/j.pvr.2018.10.004